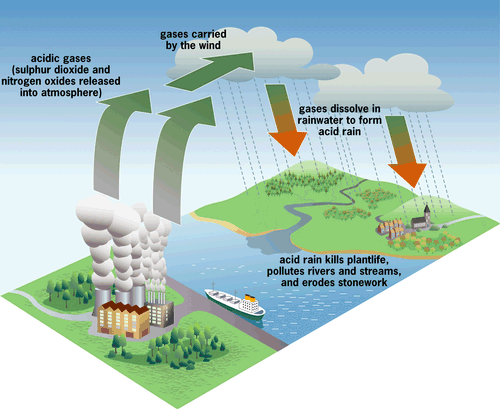
Acid rain is a result of air pollution. When any type of fuel is burnt, lots of different chemicals are produced. The smoke that comes from a fire or the fumes that come out of a car exhaust don't just contain the sooty grey particles that you can see - they also contains lots of invisible gases that can be even more harmful to our environment.
Power stations, factories and cars all burn fuels and therefore they all produce polluting gases. Some of these gases (especially nitrogen oxides and sulphur dioxide) react with the tiny droplets of water in clouds to form sulphuric and nitric acids. The rain from these clouds then falls as very weak acid - which is why it is known as "acid rain".
How acidic is acid rain?
Acidity is measured using a scale called the pH scale. This scale goes from 0 to 14. 0 is the most acidic and 14 is the most alkaline (opposite of acidic). Something with a pH value of 7, we call neutral, this means that it is neither acidic nor alkaline.
Very strong acids will burn if they touch your skin and can even destroy metals. Acid rain is much, much weaker than this, never acidic enough to burn your skin.
Rain is always slightly acidic because it mixes with naturally occurring oxides in the air. Unpolluted rain would have a pH value of between 5 and 6. When the air becomes more polluted with nitrogen oxides and sulphur dioxide the acidity can increase to a pH value of 4. Some rain has even been recorded as being pH2.
Vinegar has a pH value of 2.2 and lemon juice has a value of pH2.3. Even the strongest recorded acid rain is only about as acidic as lemon juice or vinegar and we know that these don't harm us - so why do we worry about acid rain?
The Effects of Acid Rain
Acid rain can be carried great distances in the atmosphere, not just between countries but also from continent to continent. The acid can also take the form of snow, mists and dry dusts. The rain sometimes falls many miles from the source of pollution but wherever it falls it can have a serious effect on soil, trees, buildings and water.
Forests all over the world are dying, fish are dying. In Scandinavia there are dead lakes, which are crystal clear and contain no living creatures or plant life. Many of Britain's freshwater fish are threatened, there have been reports of deformed fish being hatched. This leads to fish-eating birds and animals being affected also. Is acid rain responsible for all this? Scientists have been doing a lot of research into how acid rain affects the environment.
Forests
It is thought that acid rain can cause trees to grow more slowly or even to die but scientists have found that it is not the only cause. The same amount of acid rain seems to have more effect in some areas than it does in others.
As acid rain falls on a forest it trickles through the leaves of the trees and runs down into the soil below. Some of it finds its way into streams and then on into rivers and lakes. Some types of soil can help to neutralise the acid - they have what is called a "buffering capacity".

Other soils are already slightly acidic and these are particularly susceptible to the effects of acid rain.
Acid rain can effect trees in several different ways, it may:
• dissolve and wash away the nutrients and minerals in the soil
which help the trees to grow.
• cause the release of harmful substances such as aluminium into the soil.
• wear away the waxy protective coating of leaves, damaging them
and preventing them from being able to photosynthesise properly.
A combination of these effects weakens the trees which means that they can be more easily attacked by diseases and insects or injured by bad weather. It is not just trees that are affected by acid rain, other plants may also suffer.
Lakes and Rivers
It is in aquatic habitats that the effects of acid rain are most obvious. Acid rain runs off the land and ends up in streams, lakes and marshes - the rain also falls directly on these areas.
As the acidity of a lake increases, the water becomes clearer and the numbers of fish and other water animals decline. Some species of plant and animal are better able to survive in acidic water than others. Freshwater shrimps, snails, mussels are the most quickly affected by acidification followed by fish such as minnows, salmon and roach. The roe and fry (eggs and young) of the fish are the worst affected, the acidity of the water can cause deformity in young fish and can prevent eggs from hatching properly.
The acidity of the water does not just affect species directly, it also causes toxic substances like aluminium to be released into the water from the soil, harming fish and other aquatic animals.
Lakes, rivers and marshes each have their own fragile ecosystem with many different species of plants and animals all depending on one another to survive. If a species of fish disappears, the animals which feed on it will gradually disappear too. If the extinct fish used to feed on a particular species of large insect, that insect population will start to grow, this in turn will affect the smaller insects or plankton on which the larger insect feeds.
Buildings
Every type of material will become eroded sooner or later by the effects of the climate. Water, wind, ice and snow all help in the erosion process but unfortunately, acid rain can help to make this natural process even quicker. Statues, buildings, vehicles, pipes and cables can all suffer. The worst affected are things made from limestone or sandstone as these types of rock are particularly susceptible and can be affected by air pollution in gaseous form as well as by acid rain.
Where is it coming from?
Until relatively recently air pollution has been seen as a local issue. It was in southern Scandinavia in the late 1950's that the problems of acid rain were first observed and it was then that people began to realise that the origins of this pollution were far away in Britain and Northern Europe. One early answer to industrial air pollution was to build very tall chimneys. Unfortunately all this does is push the polluting gases up into the clouds allowing emissions to float away on the wind. The wind carries the pollution many hundreds of miles away where it eventually falls as acid rain. In this way Britain has contributed at least 16% of the acid deposition in Norway. Over ninety percent of Norway's acid pollution comes from other countries. The worst European polluters are Germany, UK, Poland and Spain, each of them producing over a million tons of sulphur emissions in 1994. Governments are now beginning to admit that acid rain is a serious environmental problem and many countries are now taking steps to reduce the amount of sulphur and nitrogen emissions.
What can be done?
Reduce emissions:
• Burning fossil fuels is still one of the cheapest ways to produce electricity so people are now researching new ways to burn fuel which don't produce so much pollution.
• Governments need to spend more money on pollution control even if it does mean an increase in the price of electricity.
• Sulphur can also be 'washed' out of smoke by spraying a mixture of water and powdered limestone into the smokestack.
• Cars are now fitted with catalytic converters which remove three dangerous chemicals from exhaust gases.
Find alternative sources of energy
• Governments need to invest in researching different ways to produce energy.
• Two other sources that are currently used are hydroelectric and nuclear power. These are 'clean' as far as acid rain goes but what other impact do they have on our environment?
• Other sources could be solar energy or windmills but how reliable would these be in places where it is not very windy or sunny?
• All energy sources have different benefits and costs and all theses have to be weighed up before any government decides which of them it is going to use.
Conserving Resources
• Greater subsidies of public transport by the government to encourage people to use public transport rather than always travelling by car.
• Every individual can make an effort to save energy by switching off lights when they are not being used and using energy-saving appliances - when less electricity is being used, pollution from power plants decreases.
• Walking, cycling and sharing cars all reduce the pollution from vehicles

Restoring the Damage done by Acid Rain
Lakes and rivers can have powdered limestone added to them to neutralise the water - this is called "liming". Liming, however, is expensive and its effects are only temporary - it needs to be continued until the acid rain stops. The people of Norway and Sweden have successfully used liming to help restore lakes and streams in their countries. A major liming programme is currently taking place in Wales.
 RSS Feed
RSS Feed Twitter
Twitter